The use of elevated mean arterial pressure (MAP) to help manage spinal cord injury has been a mainstay of treatment for years. The concept is similar to that used for management of severe traumatic brain injury. The theory is that there may be areas of the brain that are damaged, but not irretrievably so. Increasing MAP should improve perfusion and may protect areas in jeopardy from secondary injury.
As with so much in neurotrauma, few large and/or prospective studies exist. Although most centers have specific algorithms and MAP goals, optimal treatment still needs to be determined.
EAST sponsored a prospective, multicenter study to identify factors influencing neurologic outcomes after spinal cord injury. MAP augmentation was monitored, specifically its impact on the American Spinal Injury Association (ASIA) score between admission and discharge.
The ASIA Score is calculated by performing a very detailed exam consisting of myotomal motor function, a dermatomal sensory exam, and an anorectal exam. The exam takes quite some time to complete. The copy of the worksheet below should give you an idea of the level of detail:
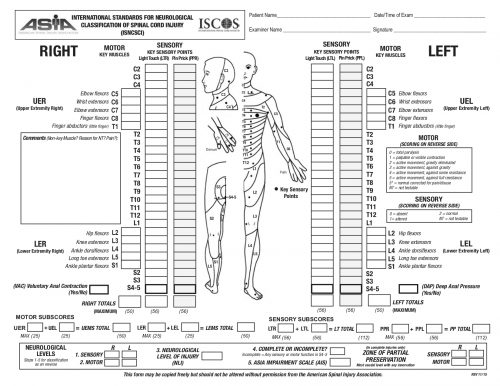
The study was performed over 20 months, and 19 centers participated. They entered 222 patients, but only 164 had pre- and post-ASIA scores for comparison.
Here are the factoids:
- Of the 164 patients studied, only 36 improved vs. 128 that showed no improvement by ASIA score
- Demographics, hospital and ICU length of stay, and mortality were not significantly different between the groups
- ISS was nearly identical (23 vs 25)
- Three-quarters of injuries were to the cervical spine, about 10% to the lumbar spine, and the remainder to the thoracic spine. There was no correlation between injury location and recovery.
- Presentation in the trauma bay (blood pressure, pulse, MAP, lactate, and Hgb) were the same in both groups
- The MAP goal of >85 mm Hg was met about 75% of the time in both groups
- Duration of MAP therapy was the same for the two groups, from 99-113 hours
- There was a trend toward increased cardiac issues (atrial fibrillation, v-tach, elevated troponin) in the group with improved spinal cord recovery. This may be due to the medications used to increase MAP.
Bottom line: This is very interesting work and will make us question the utility of MAP therapy for spinal cord injury. However, this is not a cut-and-dried conclusion. Here are several things that come to mind:
- What was the definition of “improvement?” ASIA is a complicated scoring system with many steps in the evaluation. Usually, the results are condensed into an overall “ASIA Impairment Scale,” or AIS.
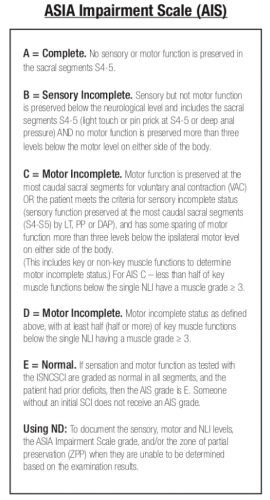 The AIS is not very granular, meaning that each step in the scale represents a large difference in function. Could patients have had improvements that did not change the AIS score but were functionally significant for the patient? For example, an improvement from a C5 to a C6 level makes a big difference in daily activities.
The AIS is not very granular, meaning that each step in the scale represents a large difference in function. Could patients have had improvements that did not change the AIS score but were functionally significant for the patient? For example, an improvement from a C5 to a C6 level makes a big difference in daily activities. - Was the study large enough? It is difficult to accumulate a large series of spinal cord injury patients. Combining this point with the previous one, was the statistical power present even to detect a meaningful difference in the AIS?
- Was MAP>85 torr maintained reliably and for long enough? Patients had MAP therapy for just over four days, and it was only maintained above the threshold about 75% of the time. We have good evidence in the brain injury literature that a single bout of hypotension in patients with severe TBI significantly increases mortality. Could it be that maintaining increased spinal cord perfusion is equally important? Could a single low MAP cause damage? It would be interesting to see if patients who had very consistent MAP therapy, say greater than 90% or 95% of the time, had any difference in outcomes. Unfortunately, I suspect that the numbers would be far too low to prove anything.
This abstract brings up some interesting questions. However, I would not consider throwing out the use of MAP goals based on it. We need more patients to study and be better at applying this treatment if we hope to uncover whether it really works.
Reference: Does mean arterial pressure augmentation improve neurological recovery of blunt spinal cord injuries: an EAST multicenter trial. EAST 2024 Podium paper #1.


 (TEG MA for liquid (LQP) and thawed (TP) plasma
(TEG MA for liquid (LQP) and thawed (TP) plasma