Falls are by far the most common mechanism of injuries in US trauma centers these days. They typically occur in elderly patients, and a growing number are on some type of oral anticoagulant for their medical conditions. And the number of these patients who are taking a DOAC (direct thrombin inhibitor or factor Xa antagonist) is rising quickly.
Unfortunately, most of the DOACs do not have good reversal agents, and they are very, very expensive. Specifically, Andexanet Alfa, the antidote for rivaroxaban and apixaban used to cost in excess of $50,000 per dose. This has come down over time to “only” $22,000 per dose. Unfortunately, the half-life is much shorter than the agent it is neutralizing, frequently requiring two doses. And the kicker is that there are no studies definitively showing that Andexanet Alfa improves mortality when used for CNS hemorrhage.
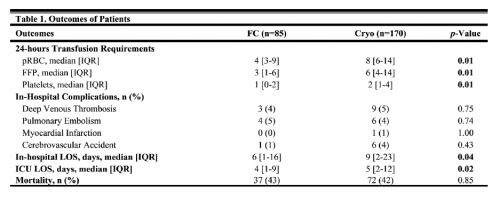
Prothrombin complex concentrate (PCC) has been used for reversal of these agents as well. Its efficacy is also not well known. The group at George Washington University is presenting an abstract comparing it against Andexanet Alfa (AA) for reversal of either of the Factor Xa inhibitors (rivaroxaban, apixaban). They performed a multicenter study involving 10 trauma centers. The endpoints studied were number of transfusions, mortality, and ICU length of stay.
Here are the factoids:
- From a total of 263 patients, 77 received AA and 186 received PCC
- Only 4% of patients received a second dose of AA despite its short half-life
- There was no significant difference in the number of PRBCs transfused
- The authors stated that the mortality was significantly lower with PCC but the p value in the data table provided was = 0.05
- They also stated that the ICU LOS was significantly lower with PCC (1.2 vs 1.5 days, p = 0.04)
The authors concluded that PCC is non-inferior to AA for reversal in bleeding trauma patients. They recommended a randomized study be done.
Bottom line: The first thing for you to know is that I have never been impressed with the data on Andexanet Alfa. Which means I have to be very careful and aware of my own cognitive bias. In practice, this means I can’t just look at the study title or abstract and be happy that it meets my confirmation bias. I have to make a conscious effort to critically read the paper or abstract and see if it really does mean what I want it to mean, or if I need to change my opinion.
This abstract doesn’t really satisfy my confirmation bias. The title states that PCC is not inferior to AA. I would certainly like to believe that. But in order to safely say that, it is vitally important that a power analysis is performed to ensure that enough patients are present in both treatment groups to confidently state that there was no difference. If the number of patients is too small, significance can’t be detected and non-inferiority cannot be confirmed.
The body of the abstract claims that mortality was significantly lower in the PCC group, although the table states that the p value was 0.05, which technically is not significant. The difference in mortality numbers is impressive (PCC mortality 20% vs 32% for AA) so why the significance issue?
And one note about significance. Be careful not to conflate statistical significance with real-life significance. ICU length of stay in this study was statistically significantly shorter in the PCC group (1.2 vs 1.5 days) but I doubt that a difference of 7 hours in the ICU is clinically relevant.
Here are my questions for the authors and presenter:
- Did you have enough patients in the study to assure that the PCC treatment was actually non-inferior? Please show us your power analysis.
- What were the inclusion criteria for the study? This will help us understand the patient group better. Were these primarily head bleeds, actual external or intra-cavity hemorrhage?
- Please clarify the significance claim for mortality. The raw percentages are impressively different, but the P value is not significant.
- Could the low rate of administering a second dose of AA have influenced the outcomes? As mentioned above, the half-life of the antidote is much shorter than that of the DOAC. Perhaps giving a second dose is actually needed and could have moved the results in favor of AA.
This is a thought-provoking abstract for me. Let’s see if you can either confirm or refute my opinion on AA!
Reference: 4-FACTOR PROTHROMBIN COMPLEX CONCENTRATE IS NOT INFERIOR TO ANDEXANET ALFA FOR THE REVERSAL OF FACTOR XA INHIBITORS: AN EAST MULTICENTER STUDY. EAST 25th ASA, oral abstract #15.