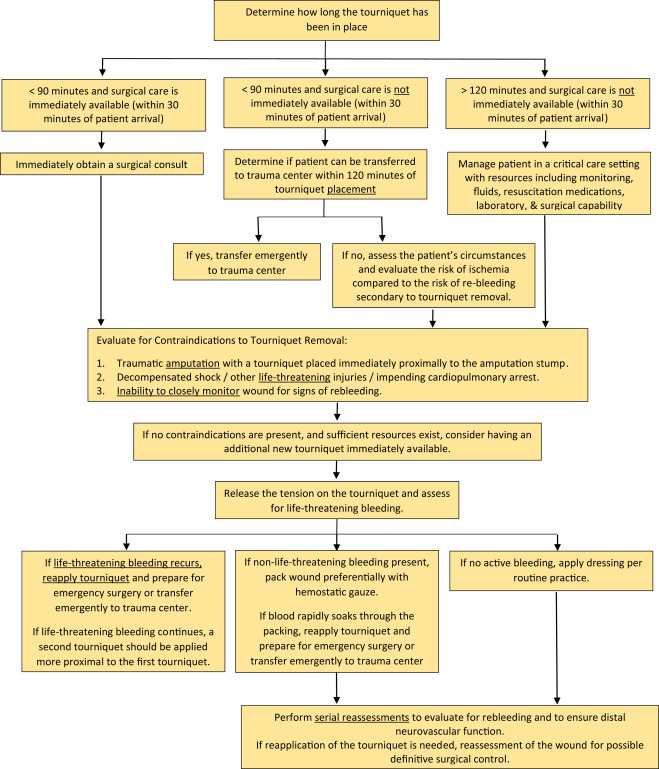
How big is too big? That has been the question for a long time as it applies to pneumothorax and chest tubes. For many, it is a math problem that takes into account the appearance on chest x-ray, the physiology of the patient, and their ability to tolerate the pneumothorax based on any pre-existing medical conditions.
I first wrote about this paper when it was just an abstract for last year’s AAST meeting. Apparently, it passed peer review muster. It has just been published in the Journal of Trauma. The numbers have changed a little bit, so I’ll update my analysis accordingly.
The group at Froedtert in Milwaukee has been trying to make the decision to place a chest tube a bit more objective. They introduced the concept of CT based size measurement using a 35mm threshold at the AAST meeting three years ago. Read my review here. My criticisms at the time centered around the need to get a CT scan for diagnosis and their subjective definition of a failure requiring chest tube insertion. The abstract never did make it to publication.
The authors are back now with a follow-on study. This time, they made a rule that any pneumothorax less than 35mm from the chest wall would be observed without tube placement. The performed a retrospective review of their experience and divided it into two time periods: 2015-2016, before the new rule, and 2018-2019, after the new rule. They excluded any chest tubes inserted before the scan was performed, those that included a sizable hemothorax, and patients placed on a ventilator or who died.
Here are the factoids:
- There were 99 patients in the early period and 167 in the later period
- Chest tube use significantly declined from 28% to 18% between the two periods. These numbers are 8% higher than were described in last year’s abstract.
- Observation rates without a chest tube increased from 85% to 95% after implementation of the new guideline
- There was no difference in length of stay, inpatient failure rate, complications, or death
- The most common inpatient failure was due to development of a new hemothorax. However, there was an almost identical number of failures of “unclear” etiology. This is troublesome but part and parcel for such a retrospective study.
- Two patients were readmitted within 30 days for a pulmonary complication (one empyema, one readmission at 3 days after discharge for dyspnea due to pneumothorax)
- Patients in the later group were 2x more likely to be observed (by regression analysis)
The authors concluded that the 35mm rule decreased unnecessary chest tube insertion while maintaining patient safety.
Bottom line: I still have a few issues with this paper and the authors’ preceding series of abstracts. First, decision to insert a chest tube required a CT scan in a patient with a pneumothorax. This seems like extra radiation for patients who may not otherwise fit any of the usual blunt imaging criteria. And, like their 2018 and 2021 abstracts, there are no objective criteria for failure requiring tube insertion. So it is difficult to gauge compliance when insertion for failure is somewhat based on the whims of the individual surgeon.
What this abstract really shows is that compliance with the new rule increased, and there were no obvious complications from its use. The other numbers (chest tube insertions, observation failure) are just too subjective to learn much from. The most troubling issue is that the reason for 40% of failures was “unclear.” This is most likely due to the fact that the authors did not have objective guidelines for failure due to the retrospective nature of the study.
The numbers in this paper changed a little from last year’s abstract. The overall conclusions and meaning did not. It appears that 35mm is a reasonable threshold for pneumothorax size when contemplating inserting a chest tube. Unfortunately, this study relied entirely on CT scan. We don’t know if using a similar guideline for regular old chest x-ray is valid or not.
What we still need is a good, prospective trial using an arbitrary guideline like 35mm pneumothorax as seen on chest x-ray or CT scan. And then, a clear definition of what defines a failure that requires tube insertion would be helpful. And at some point, we also need to know if a small tube or pigtail catheter is adequate for pure pneumothorax. Don’t get me started on that one!
Reference: The 35-mm rule to guide pneumothorax management: Increases appropriate observation and decreases unnecessary chest tubes. J Trauma 92(6):951-957, 2022.