The inferior vena cava (IVC) filter has been around in one form or another for over 40 years. One would think that we would have figured everything about it out by now. But no! The filter has evolved through a number of iterations and form factors over the years. The existing studies, in general, give us piecemeal information on the utility and safety of the device.
One of the major innovations with this technology came with the development of a removable filter. Take a look at the product below. Note the hook at the top and the (relatively) blunt tips of the feet. This allows a metal sheath to be slipped over the filter while in place in the IVC. The legs collapse, and the entire thing can be removed via the internal jugular vein.
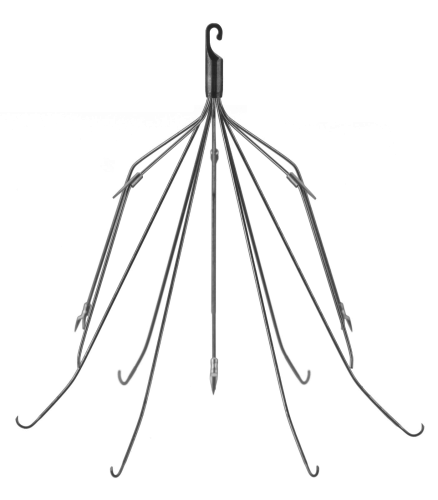
The availability of the removable filter led the American College of Chest Physicians to recommend their placement in patients with known pulmonary embolism (PE) or proximal deep venous thrombosis (DVT) in patients with contraindications to anticoagulation. Unfortunately, this has been generalized by some trauma professionals over the years to include any trauma patients at high risk for DVT or PE, but who don’t actually have them yet.
One would think that, given the appearance of one of these filters, they would be protective and clots would get caught up in the legs and be unable to travel to the lungs as a PE. Previous studies have taught us that this is not necessarily the case. Plus, the filter can’t stop clots that originate in the upper extremities from becoming an embolism. And there are quite a few papers that have demonstrated the short- and long-term complications, including clot at and below the filter as well as post-phlebitic syndrome in the lower extremities.
A study from Boston University reviewed their own experience retrospectively over a 9 year period. This cohort study looked at patients with and without filters, matching them for age, sex, race, and injury severity. The authors specifically looked at mortality, and used four study periods during the 9 year interval.
Here are the factoids:
- Over 18,000 patients were admitted during the study period, resulting in 451 with an IVC filter inserted and 1343 matched controls
- The patients were followed for an average of 4 years after hospitalization
- Mortality was identical between patients with filters vs the matched controls
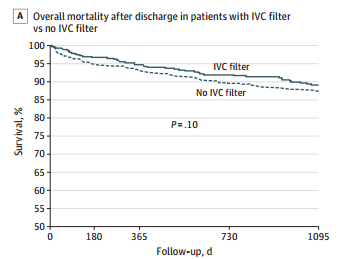
- There was still no difference in mortality, even if the patients with the filter had DVT or PE present when it was inserted
- Only 8% ever had their “removable” filter removed (!)
Bottom line: Hopefully, it’s becoming obvious to all that the era of the IVC filter has come and gone. There are many studies that show the downside of placement. And there are several (including this one) that show how forgetful we are about taking them out when no longer needed. And, of course, they are expensive. But the final straw is that they do not seem to protect our patients like we thought (hoped?) they would. It’s time to reconsider those DVT/PE protocols and think really hard about whether we should be inserting IVC filters in trauma patients at all.
Tomorrow: a look at trends in filter insertion and retrieval.
Related post:
Reference: Association Between Inferior Vena Cava Filter Insertion
in Trauma Patients and In-Hospital and Overall Mortality. JAMA Surg, online ahead of print, September 28, 2016.
